Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) subtypes have differential response to BTK inhibitors (BTKi). Ibrutinib with R-CHOP improves survival in DLBCL subsets, but toxicity is limiting. Precise characterization of BTKi-responsive tumors enhances pt selection. Acalabrutinib (acala) is a BTKi with activity in DLBCL, but the molecular correlates of acala response are unknown. Circulating tumor DNA (ctDNA) is a prognostic biomarker in DLBCL including early changes during chemotherapy. PhasED-Seq is a novel ctDNA method that lowers the error profile of mutation detection by requiring the concordant detection of two separate mutations on an individual cell-free DNA molecule (Kurtz et al. Nat Biotechnol 2021). We employed a response-adapted study of acala for up to 14d prior to frontline therapy for aggressive B-cell lymphoma to determine the molecular profile of BTKi-responsive tumors. We report preliminary results including dynamic changes in ctDNA from this ongoing trial [NCT04002947].
Methods: Pts with untreated aggressive B-cell lymphoma and any HIV status are eligible if age ≥18, ≥stage 2, PS ≤2, and adequate organ function. Pts with PMBL, unmeasurable lesions, or active CNS disease are excluded. Screening includes labs, CT and FDG-PET, BM, and CSF with flow cytometry. Pts first receive acala 100mg twice daily x 14d. Pts with <25% reduction in bi-dimensional lesions by CT after acala receive DA-EPOCH-R or R-CHOP for 4-6 cycles every 21d. Pts with ≥25% reduction on CT after the window continue acala 100mg twice daily on days 1-10 of each cycle of R-chemo. Tumors undergo multi-platform profiling including whole exome, RNA sequencing, and assessment of DNA copy number. Streck tubes are collected at baseline, after 7d of acala, end of acala, end of C2, end of therapy, and during surveillance. ctDNA levels were quantified using PhasED-Seq (Foresight Diagnostics Inc.) to track phased variants.
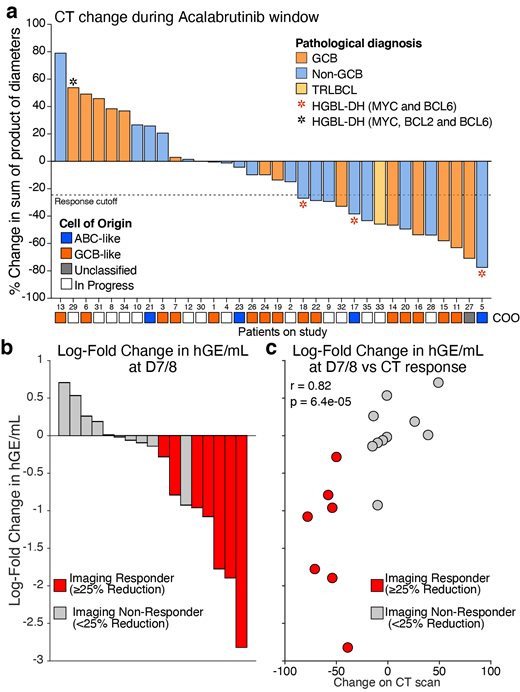
Results: 34 pts enrolled between August 2019 and July 2021 and completed the acala window. Median age was 64 (range 28-85) including 13 (38%) < 60y, 14 (41%) 61-69, and 7 (21%) ≥70y. Three (9%) pts had HIV and 17 (50%) were high-risk by IPI. The median diagnosis to treatment was 22.5d (4-53). IHC subtypes by Hans included 17 (50%) non-GCB, 16 (47%) GCB, and 1 (3%) T-cell/histiocyte-rich large B-cell lymphoma (TRLBCL). Four (12%) pts were high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 (HGBL-DH). Fifteen (44%) pts responded to acala during the window, while 19 (56%) pts had no response (Figure 1A). Acala responses were seen across DLBCL subtypes including 7 (47%) pts with non-GCB, 7 (47%) pts with GCB, and 1 (7%) pt with TRLBCL. Twenty pts had RNA sequencing to confirm cell-of-origin including 10 responders which included 7 (70%) GCB, 2 (20%) ABC, and 1 (10%) Unclassified. Notably, 13 (86%) BTKi-responsive tumors were CD10 negative and only 2 (18%) CD10+ tumors were BTKi-responsive. ctDNA dynamics strongly correlated with CT response as the log-fold change in ctDNA (hGE/mL) at the end of the window correlated with change on CT (r=0.75, p=0.0013). Remarkably, ctDNA dynamics after only 7d also correlated with change on CT (r=0.82, p=0.00006)(Figure 1B-C). Interestingly, one pt had improved symptoms and a 20-fold drop in ctDNA, but no corresponding CT changes suggesting that ctDNA changes may precede CT changes in some cases. Twenty-nine (85%) pts completed all planned cycles of therapy while 5 pts stopped chemotherapy early due to myelosuppression (n=3), CHF (n=1), and MI (n=1). Toxicity across 156 cycles was mostly hematologic. G3/G4 neutropenia occurred in 50% and 38% of cycles and febrile neutropenia in 10% of cycles. G3/G4 thrombocytopenia occurred in 22% and 12% of cycles. No increase in infections, atrial fibrillation, or bleeding were observed in pts treated with acala. All 27 pts who completed therapy achieved a CR. Two pts (1 acala responder) have relapsed from CR and 1 pt died of an MI. After a median follow-up of 9.2m the estimated 1-year PFS was 84.9% (95% CI: 58-95).
Conclusions: Acalabrutinib prior to frontline therapy has activity in GCB, non-GCB, and HGBL-DH: confirmed by gene expression profiling. CD10+ GCB tumors are mostly acala-resistant. Toxicity is mainly hematologic and manageable across age groups including pts with HIV. ctDNA correlates with CT change and may predict response to targeted agents as early as 7 days. Updated clinical results within genetic subtypes will be presented at the meeting.
Chabon: Foresight Diagnostics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Lurain: CTI Biopharma: Research Funding; EMD-Serrono: Research Funding; Merck: Research Funding; BMS-Celgene: Research Funding; Janssen: Research Funding. Bagaev: BostonGene Corp.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: BostonGene. Postovalova: BostonGene Corp.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: BostonGene. Meerson: BostonGene: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: BostonGene. Kudryashova: BostonGene: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: BostonGene. Kotlov: BostonGene Corp: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties. Fowler: BostonGene: Current Employment, Current holder of stock options in a privately-held company. Kurtz: Roche: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech: Consultancy. Alizadeh: CAPP Medical: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Forty Seven: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Foresight Diagnostics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Roche: Consultancy, Honoraria; Janssen Oncology: Honoraria; Celgene: Consultancy, Research Funding; Gilead: Consultancy; Bristol Myers Squibb: Research Funding; Cibermed: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal